46++ How Do You Find The Empirical Formula Of Magnesium Oxide ideas
How do you find the empirical formula of magnesium oxide. To work out the empirical formula the value of moles of the different atoms in a compound is needed. The empirical formula is the simplest and lowest whole number ratio of the different atoms in a sample of compound. Describe an experiment to determine the empirical formula of a simple compound such as magnesium oxide. To find the empirical formula we reduce the CH atom ratio to the smallest whole-number ratio as shown below. If you are trying to find the empirical formula for a homework assignment you will most likely be given the percentage compositionyou just need to know where to look. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test. To find out the empirical formulae for magnesium-oxide Introduction. The empirical formula does not tell us the actual number of atoms in the molecule. Chemistry Investigation to find the Empirical Formula of Magnesium Oxide Pages. The empirical formula of a compound tells us the types of atoms present in a compound as well as the simplest whole-number ratio of the different types of atoms. From the combination of chemical properties between Magnesium and Oxygen we can absolutely calculate the empirical formula Magnesium Oxide we measure the mass of the Magnesium initially and the mass of Magnesium Oxide yet we can just find the difference between Magnesium Oxide and Magnesium to get the mass.
You will synthesize this compound by heating a sample of magnesium strongly in the presence of oxygen. In the Lab Determining the Empirical Formula of Magnesium Oxide students set out to find if there is a true 11 ratio in the empirical formula of MgO. To find the empirical formula for magnesium oxide we must first find the molecular formula to identify how many of each elements atoms are in the. Heat using blue flame until Magnesium burns. How do you find the empirical formula of magnesium oxide DiscussionThe independent variable is the amount of magnesium and the dependent variable is the mass of magnesium oxide. This showed the reaction of Oxygen combining with Magnesium to form Magnesium Oxide. Finding the empirical formula of a metal oxide The empirical formula of magnesium oxide can be calculated using the following experiment which finds the. Find Formula Divide each by the smallest number to get a whole number ratio for the formula. Knowing the mass of magnesium used and the mass of magnesium oxide produced you. How to find the formula of magnesium oxide using an unbreakable crucible. Empirical formulaof a compound gives the lowest whole number ratio of atoms of each element present in the compound. Find Moles Convert grams of Mg to moles03702mol Convert grams of O to moles. The mass of Mg the mass of O2mass of MgxOx.
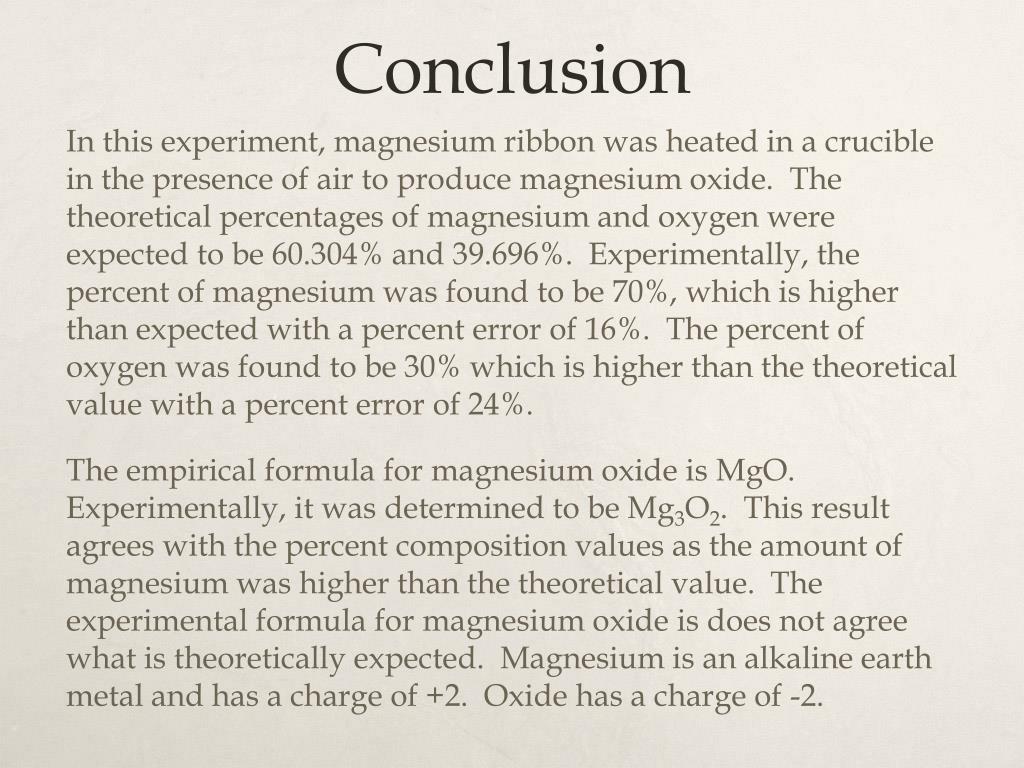 Ppt Determination Of The Empirical Formula Of Magnesium Oxide Exemplar Powerpoint Presentation Id 1910092
Ppt Determination Of The Empirical Formula Of Magnesium Oxide Exemplar Powerpoint Presentation Id 1910092
How do you find the empirical formula of magnesium oxide 4 964 words Magnesium Oxide Chemistry Report Pages.

How do you find the empirical formula of magnesium oxide. Allow to cool then record mass. In todays lab you will experimentally determine the empirical formula of a compound known as magnesium oxide. This was determined by burning the Magnesium until a white smoke started to protrude.
And the University of California Santa Cruz 2. See full answer below. This is found by determining the moles c 2011-2015 Advanced Instructional Systems Inc.
MgO 2 2 Determine the theoretical formula of MgO using the periodic table. 5 1482 words To determine the standard enthalpy of formation of Magnesium Oxide. Results ObjectMass Therefore empirical formula is Mg5O14.
Lets say that the assignment asks you. Determining the Empirical Formula of Magnesium Oxide INTRODUCTION. Magnesium Oxygen Magnesium Oxide.
The empirical formula of magnesium oxide Mg xO yis written as the lowest whole-number ratio between the moles of Mg used and moles of O consumed. 3 885 words Empirical Formula Lab Report Pages. Empirical formula of magnesium oxide is determined by reacting magnesium metal with oxygen from the air to produce the magnesium oxide.
Through simple conversion of grams to moles the molar ratio of magnesium to oxygen can be determined and the formula predicted. 10 2786 words Finding thr Percentage Composition of Magnesium Oxide Pages. The objective of the experiment is to determine the empirical formula of Magnesium Oxide through a procedure of heating magnesium ribbon to react with oxygen to form a magnesium oxide compound with the correct ratio of atoms within each element.
Replace lid and heat for ten minutes.
How do you find the empirical formula of magnesium oxide Replace lid and heat for ten minutes.
How do you find the empirical formula of magnesium oxide. The objective of the experiment is to determine the empirical formula of Magnesium Oxide through a procedure of heating magnesium ribbon to react with oxygen to form a magnesium oxide compound with the correct ratio of atoms within each element. 10 2786 words Finding thr Percentage Composition of Magnesium Oxide Pages. Through simple conversion of grams to moles the molar ratio of magnesium to oxygen can be determined and the formula predicted. Empirical formula of magnesium oxide is determined by reacting magnesium metal with oxygen from the air to produce the magnesium oxide. 3 885 words Empirical Formula Lab Report Pages. The empirical formula of magnesium oxide Mg xO yis written as the lowest whole-number ratio between the moles of Mg used and moles of O consumed. Magnesium Oxygen Magnesium Oxide. Determining the Empirical Formula of Magnesium Oxide INTRODUCTION. Lets say that the assignment asks you. Results ObjectMass Therefore empirical formula is Mg5O14. 5 1482 words To determine the standard enthalpy of formation of Magnesium Oxide.
MgO 2 2 Determine the theoretical formula of MgO using the periodic table. This is found by determining the moles c 2011-2015 Advanced Instructional Systems Inc. How do you find the empirical formula of magnesium oxide See full answer below. And the University of California Santa Cruz 2. This was determined by burning the Magnesium until a white smoke started to protrude. In todays lab you will experimentally determine the empirical formula of a compound known as magnesium oxide. Allow to cool then record mass.
 Empirical Formula Of Magnesium Oxide Kcpc Org
Empirical Formula Of Magnesium Oxide Kcpc Org